Background
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by reduced platelet counts. Patients with ITP lasting for ≥3 months (persistent or chronic primary ITP), and who do not respond adequately or maintain a response to initial therapy, are usually treated with advanced therapies such as thrombopoietin receptor agonists (TPO-RAs [avatrombopag/romiplostim/eltrombopag]), rituximab, and fostamatinib. The economic and clinical outcomes among patients on advanced therapies for ITP are not well described in real-world data.
Objective
This study aimed to evaluate the clinical and economic burden among ITP patients (lasting ≥3 months) treated with TPO-RAs, or rituximab, or fostamatinib by therapy type.
Methods
This retrospective cohort study used the US administrative health insurance claims data from the Optum's de-identified Clinformatics ® Data Mart Database. Patients with ITP lasting ≥3 months (aged ≥18 years), treated with TPO-RAs, or rituximab, or fostamatinib (index) between October 1, 2015, to August 31, 2020, after a 180-days washout period were included. Patient characteristics were evaluated 180 days prior to index (baseline). Follow-up for clinical outcomes started one-day after the index date and on the index date for economic outcomes. Patients were censored on switch to another ITP therapy different from index, death, end of data, or end of enrollment. Clinical outcomes (bleeding events [overall and type of bleeding], thromboembolic events [TEs], and clinical events identified as potential drug related adverse reactions) were reported as rate of events per 1000 person-years (PY). Health care resource use included number of hospitalizations, hospital days, emergency room visits, number of intensive care unit (ICU) admissions (both reported as rate of events per 1000 PY), and length of ICU stay. All analyses were stratified by treatment type on index date, however formal adjusted comparisons were not performed. Outcomes for fostamatinib users are not presented due to low sample size.
Results
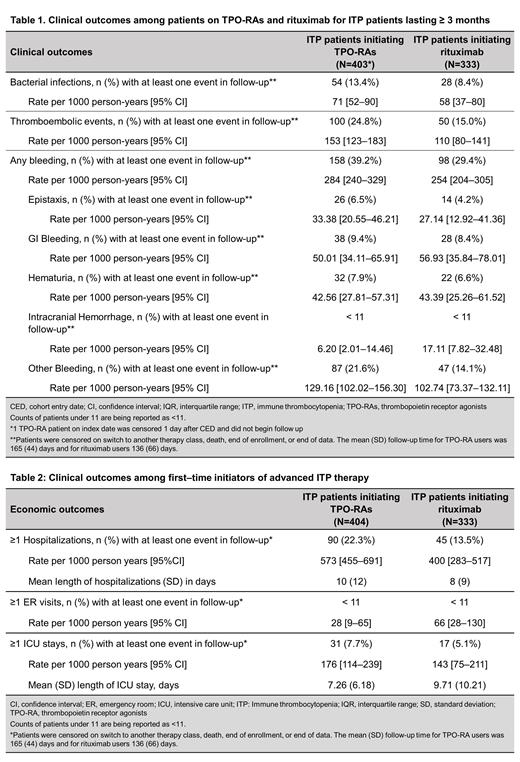
A total of 404 patients treated with TPO-RAs, 333 with rituximab, and 14 with fostamatinib were eligible for the analysis. The average age of patients across the three cohorts was 60 years; 57.0% were females. The most common comorbidities across the three cohorts were diabetes (26.8%), coronary artery disease (14.4%), chronic heart failure (9.3%), and any TE (10.5%). At baseline, 29.0% of TPO-RA users, 30.6% of rituximab users, and 28.6% of fostamatinib users had experienced bleeding events. At baseline, bacterial infections occurred in 2% to 4% of patients treated with TPO-RAs and rituximab. At baseline, 36.1% TPO-RA users, 52.9% rituximab users, and 42.9% fostamatinib users had required any rescue therapy (IV anti-D, IV immunoglobulins, IV steroids, and platelet transfusion). Patients initiating TPO-RAs and rituximab were observed for a median (interquartile range, IQR) duration of 629 (286-1077) days and 398 (75-991) days, respectively. TPO-RA users and rituximab users stayed on the therapy for a mean (SD) duration of 305 (356) days and 31 (20) days, respectively. Among rituximab users, 60.7% completed a 4-week treatment course at index.
During follow-up, the rate of bleeding events among TPO-RA and rituximab users was 284 and 254 per 1000 PY, respectively. The most common bleeding events were gastrointestinal bleeding, hematuria, and epistaxis. The rate of bacterial infections was 71 per 1000 PY in patients treated with TPO-RAs and 58 per 1000 PY in those treated with rituximab. The rate of TEs among TPO-RA and rituximab users was 153 and 110 per 1000 PY, respectively (Table 1). During follow-up, 38.4% of TPO-RA users and 55.9% of rituximab users required rescue therapy. The rate of hospitalizations among patients treated with TPO-RAs and rituximab was 573 and 400 per 1000 PY, respectively. The rate of ICU stays in patients treated with TPO-RAs was 176 per 1000 person-years, and 143 per 1000 person-years for those treated with rituximab (Table 2).
Conclusion
ITP patients (lasting ≥ 3 months) treated with TPO-RAs and rituximab continue to experience substantial rates of infections, TEs and bleeding (often requiring rescue therapies). All-cause hospitalization was high in this population. Further studies are needed to understand long-term clinical and economic outcomes among ITP patients.
Disclosures
Kuter:AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui. Immunovant, Incyte, Inmagenebio: Consultancy; AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui, Immunovant, Incyte, Inmagenebio, Ke: Honoraria; Rubius: Current equity holder in publicly-traded company; UpToDate: Patents & Royalties: UpToDate Chapters; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Kezar, Kyowa-Kirin, Merck Sharp & Dohme: Honoraria; Kezar, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Nuvig, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Protagonist, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, Up-To-Date, Zafge: Consultancy; Alnylam, BioCryst, Novartis, Rigel, Sanofi (Principia), Takeda (Bioverativ), and UCB: Research Funding. Umarje:Sanofi: Current Employment. Gouia:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Cordoba:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Ward:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Hemim:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Petruski-Ivleva:Sanofi: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal